The ability of certain Antarctic fish to survive in subzero temperatures has long fascinated scientists. Among their remarkable adaptations is a unique protein found in their corneas that prevents ice crystal formation. This protein, known as antifreeze glycoprotein (AFGP), has become a focal point of research in materials science and cryobiology. Its molecular structure holds secrets that could revolutionize technologies ranging from organ preservation to aerospace engineering.
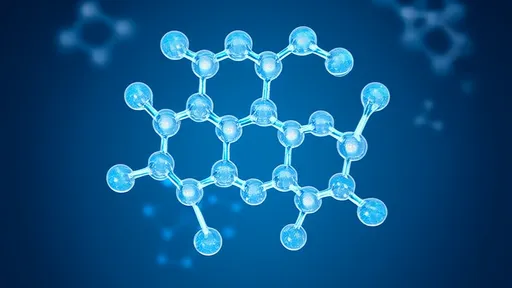
At the heart of this biological marvel lies a simple yet elegant molecular design. AFGPs consist of repeating tripeptide units (Ala-Ala-Thr) with a disaccharide attached to each threonine residue. This structure creates a surface that binds to nascent ice crystals, inhibiting their growth through a process called adsorption inhibition. The protein doesn't lower freezing temperature in the traditional sense but rather prevents microscopic ice crystals from developing into dangerous shards that could damage tissues.
What makes these proteins extraordinary is their specificity. Unlike chemical antifreeze agents that work through colligative properties, AFGPs operate at concentrations thousands of times lower. A mere 1 mg/mL of AFGP can depress the freezing point by 0.5°C without significantly affecting the melting point - a phenomenon termed thermal hysteresis. This selective action prevents the protein from disrupting normal cellular functions while providing targeted protection against ice formation.
The three-dimensional configuration of AFGPs reveals an amphipathic nature crucial to their function. X-ray crystallography and NMR studies show that the sugar moieties form a hydrophilic face that interacts with ice lattice structures, while the peptide backbone creates a hydrophobic surface facing the liquid phase. This dual nature allows the protein to simultaneously engage with both ice and water molecules, creating a physical barrier to crystal growth.
Evolution has fine-tuned these proteins over millions of years. Different Antarctic species produce slightly varied AFGP isoforms, each optimized for their specific environmental niche. The notothenioid fish, for instance, produces at least eight distinct AFGP variants with molecular weights ranging from 2.6 to 33.7 kDa. This diversity suggests an evolutionary arms race between the fish's need to prevent freezing and the physical constraints of protein synthesis in cold environments.
Recent advances in synthetic biology have enabled researchers to produce recombinant AFGPs, opening new avenues for practical applications. Scientists have successfully expressed these proteins in bacteria, yeast, and even plants. However, reproducing the exact post-translational modifications - particularly the glycosylation patterns - remains challenging. The β-galactosyl-(1→3)-α-N-acetylgalactosamine disaccharide appears crucial for full activity, yet synthetic pathways often fail to replicate this precisely.
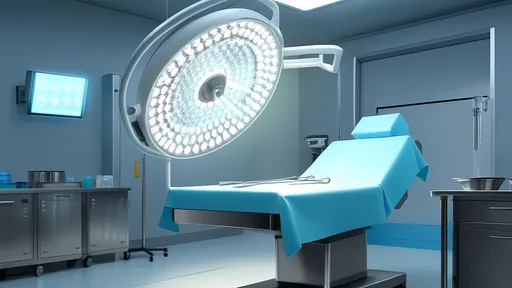
The potential applications of AFGP technology span multiple industries. In cryopreservation, incorporating these proteins could dramatically improve organ and tissue storage. Current vitrification methods often cause ice damage during thawing, but AFGPs might enable true supercooling without crystallization. Food science stands to benefit as well - imagine ice cream that resists freezer burn or frozen dough that maintains perfect texture after thawing.
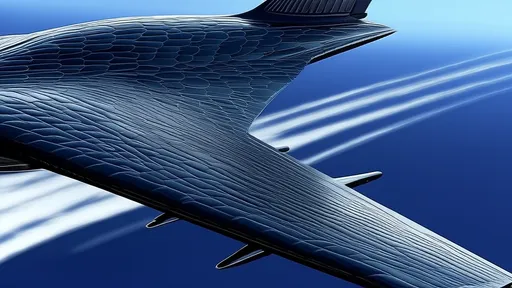
Perhaps most intriguing are the biomimetic materials inspired by AFGPs. Researchers have developed synthetic polymers that mimic the ice-binding surface of these proteins. These materials show promise for preventing ice accumulation on aircraft wings, wind turbines, and power lines. Early prototypes demonstrate that even partial replication of the protein's structure can achieve significant anti-icing effects at temperatures far below traditional de-icing agents' operational limits.
However, significant challenges remain before widespread commercialization. The cost of producing pure AFGPs remains prohibitive for most applications. Moreover, the long-term stability of these proteins outside their native environment requires further study. Some researchers worry about potential ecological impacts if synthetic AFGPs were to enter natural water systems, possibly disrupting microbial ecosystems adapted to seasonal freeze-thaw cycles.
Ongoing research continues to uncover new facets of these remarkable proteins. Recent studies suggest that AFGPs may have secondary functions beyond freeze protection, including stabilization of cell membranes under cold stress. Other investigations explore whether similar proteins exist in Arctic species or whether the Antarctic variants evolved independently. Each discovery adds another piece to the puzzle of how life adapts to extreme environments.
The story of antifreeze proteins reminds us that nature often develops solutions far more elegant than human engineering. As we face growing challenges from climate change and technological demands, looking to polar fish for inspiration might provide unexpected answers. The humble AFGP, perfected over eons in Earth's coldest waters, could well become a cornerstone of twenty-first century materials science.
By /Aug 12, 2025

By /Aug 12, 2025

By /Aug 12, 2025

By /Aug 12, 2025
By /Aug 12, 2025

By /Aug 12, 2025

By /Aug 12, 2025

By /Aug 12, 2025

By /Aug 12, 2025
By /Aug 12, 2025

By /Aug 12, 2025

By /Aug 12, 2025

By /Aug 12, 2025
By /Aug 12, 2025
By /Aug 12, 2025
By /Aug 12, 2025
By /Aug 12, 2025

By /Aug 12, 2025

By /Aug 12, 2025

By /Aug 12, 2025